Stainless steel components may degrade over time when they are exposed to internal and external factors that cause corrosion, which can cost facilities significant time and money. Deteriorated parts may require repairs or replacement, either of which can result in unplanned downtime for the system involved (Fig. 1).
Facilities should deploy components in their fluid systems made from 316 stainless steel, whose chromium levels provide extra protection against the damage corrosion can inflict.
According to the National Association of Corrosion Engineers (NACE), corrosion can cost offshore and nearshore facilities more than $1 billion each year. Fortunately, several simple solutions exist that can mitigate or eliminate the problems before they become insurmountable for oil and gas companies.
Before specific identifications are made, teams must understand what causes corrosion and what steps they can take to prevent it from worsening and putting the fluid system at risk of failure. Then maintenance teams should identify which kind of corrosion is harming their fluid systems by learning and understanding what sets the different types apart. Once a complete understanding of which corrosion is affecting a system is reached, then teams can take action.
Why stainless steel corrodes. Almost every metal in the world can corrode under certain conditions, but the harsh conditions of oil and gas applications present specific challenges especially for offshore installations.
Corrosion results from electrochemical reactions and oxidation (loss of electrons) at an anode, or reduction (gaining of electrons) at a cathode (Fig. 2). A common example is iron tubing, which may oxidize and release two electrons. Water introduced to the system can cause the iron to dissolve into Fe2 positive ions. At the same time, the electrons may cause a reduction reaction, changing dissolved O2 into OH-, a negatively charged hydroxide ion.
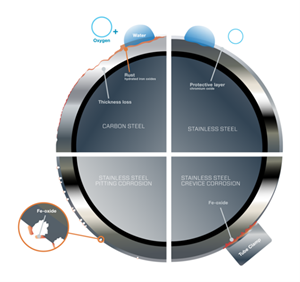
Both pitting and crevice corrosion begin when the oxide layer on the outside of the metal surface breaks down and creates space for corrosive materials to do damage.
Susceptible metal tubing can be found in analytical and process instrumentation, hydraulic lines, and control and utility applications in the oil and gas industries. To avoid initial corrosion damage, most systems are designed with stainless steel that has a minimum of 10% chromium in its composition. Sufficient chromium levels induce the creation of an oxide layer that helps slow or even prevent corrosion. Even the strongest stainless steels can succumb to corrosion if environmental conditions destroy that oxide layer. Without that layer, corrosion reactions may proliferate quickly.
The two most common corrosion types facing the oil and gas industry are pitting corrosion and crevice corrosion.
The differences between pitting and crevice corrosions. At many oil and gas facilities, several corrosion types may happen simultaneously and destroy entire fluid systems in the process because of the operation environment, materials used, and process fluids. The two most common forms of stainless steel corrosion are pitting corrosion and crevice corrosion (Fig. 3).
Pitting corrosion: Pitting occurs when the chromium layer is destroyed over time, leaving the bare metal underneath unprotected. When the bare metal is exposed to corrosive solutions, damage will occur. Typically, the damage takes the form of small cavities, commonly known as pits.

Pitting corrosion (left) and crevice corrosion (right) represent significant risks to offshore oil and gas fluid systems. Facilities should make sure their teams are equipped with the knowledge to identify, repair, and prevent either type of corrosion.
Visual inspection may reveal the beginnings of pitting corrosion, but the amount of lost material underneath the surface can silently undermine a pipe’s performance. In the worst-case scenarios, pitting corrosion can perforate tube walls and result in costly leaks. Left unaddressed, pitting corrosion can lead to cracks in components under tensile loads. If the environment contains high levels of chloride (CI-), such as seawater hitting offshore drilling platforms, pitting corrosion is even more likely, especially if high temperatures are involved.
A clear signal that pitting corrosion is occurring is reddish-brown iron oxide deposits on the surface as well as the beginnings of actual pits. If CI-bearing water like seawater pools and evaporates, the remaining solution will become even more corrosive. Keep upward-facing surfaces clear of standing water to prevent CI-induced pitting corrosion.
Crevice corrosion: The cause of crevice corrosion is similar to pitting corrosion, meaning that the oxide layer has broken down. What makes crevice corrosion more insidious is that it rarely happens in plain sight. Instead, as the name implies, it occurs in crevices, making it more challenging to find and prevent. Additionally, the wide and relatively shallow pits that occur in crevice corrosion only grow once the process has begun. In most fluid systems, crevices are created between tubing and tube supports or clamps, between adjacent tubing runs, and under dirt and deposits that have collected on the surface.
No matter how clever the design, crevices will inevitably happen. The tightest crevices pose the single greatest danger to the integrity of the system’s stainless steel components. Crevices are particularly problematic in offshore applications because seawater can diffuse into the crevice without access to an outlet. The resulting chemically aggressive environment does not allow corrosion-causing ions to come back out, leaving the entire surface susceptible to rapid corrosion.
Additionally, crevice corrosion often stays hidden until a tubing clamp is removed, leaving it potentially undetected for long periods of time. Unlike pitting corrosion, crevice corrosion occurs at lower temperatures because it is easier to create a pit beneath a tube clamp or other such device.
Keeping corrosion from occurring. The simplest way to minimize corrosion is to educate maintenance teams about basic materials knowledge and instituting corrosion-prevention standards for the facility.
First, consider the choice of materials for tubing applications, from the tubing itself to tube supports and clamps. Laboratory testing for critical pitting temperature (CPT) and critical crevice temperature (CCT)—according to the ASTM G48 standard—is an invaluable tool for comparing materials to be used in corrosive environments. CPT testing evaluates the temperature at which pitting begins on a material in a specific corrosive solution. Similarly, CCT testing evaluates at what temperature crevice corrosion begins when a predefined crevice is placed on a metal sample in a corrosive solution.
Materials that have high values for CPT and CCT are generally more suitable for use in similar corrosive environments than materials with low values. For example, 304L has the lowest CPT value of the materials shown in (Fig. 4) while 6Mo and 2507 have the two highest CPT and CCT values. This suggests that 6Mo and 2507 are likely to be more resistant to pitting and crevice corrosion than 304L and 316L in chloride-bearing solutions. It is important to keep in mind that these tests are useful for comparison and material selection but are not predictive of when a material will fail in a real-life application.
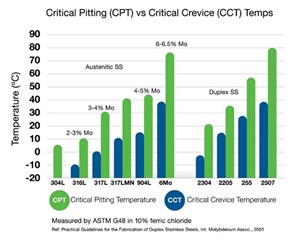
Critical Pitting Temperatures (CPT) and Critical Crevice Temperature (CCT) are important values used to determine which materials will best resist corrosion in harsh operating environments.
The 316L grade of stainless steel (UNS S31603) tubing works well in many installations as long as it is kept clean, and temperatures are not excessively high. In warmer climates, especially in locations where salt deposits readily form and in installations where rust from carbon steel structural beams and floors accumulates on stainless steel surfaces, corrosion of 316L stainless steel tubing is more readily observed. However, due to the beneficial addition of molybdenum, 316L typically performs better than 304L (UNS S30403) stainless steel in these corrosive environments.
For situations where 316L is insufficient to meet the lifetime requirements of the application, tubing made from super austenitic (e.g., 6Mo or 6HN, UNS N08367) or super duplex (e.g., 2507, UNS S32750) stainless steels offers significantly improved corrosion resistance. Additionally, the higher yield and tensile strength of super austenitic and super duplex stainless steels make it easier to build systems that must be rated to a higher maximum allowable working pressure (MAWP). Working with your tubing and tube fittings supplier for guidance in selecting the right products and materials can help you avoid costly errors.
In addition to materials selection, careful system practices are necessary for preventing corrosion and minimizing the number of locations where crevice corrosion can occur. One way to mitigate crevice corrosion in a tube system is to avoid placing tubing directly against walls or against each other. When crevice corrosion of 316L stainless steel tubing is observed, one can replace 316L tubing with more corrosion-resistant tubing such as 6Mo, which can be installed with cost-effective 316L tube fittings in suggested mixed-material engineered combinations.
Why it matters. Oil and gas companies are increasingly under pressure to keep maintenance costs down, and maintaining fluid systems at peak performance is an essential way to help accomplish it. In reality, that means making sure the maintenance team understands how to identify, correct, and prevent corrosion before it damages an entire system.
Building a basic understanding of corrosion—what it looks like, where it occurs, and for what reasons—among those who regularly work with tubing systems can help prevent material failure and costly repairs as well as improve system longevity.
This article was originally posted at www.worldoil.com



Be the first to comment